COVID-19: Impact in endothelial function and therapy with Mesenchymal Stromal Cells
DOI:
https://doi.org/10.54502/msuceva.v1n1a2Palabras clave:
COVID-19 pathophysiology, Endothelial disfunction, Immune cells, Mesenchymal Stromal Cells, PathogenesisResumen
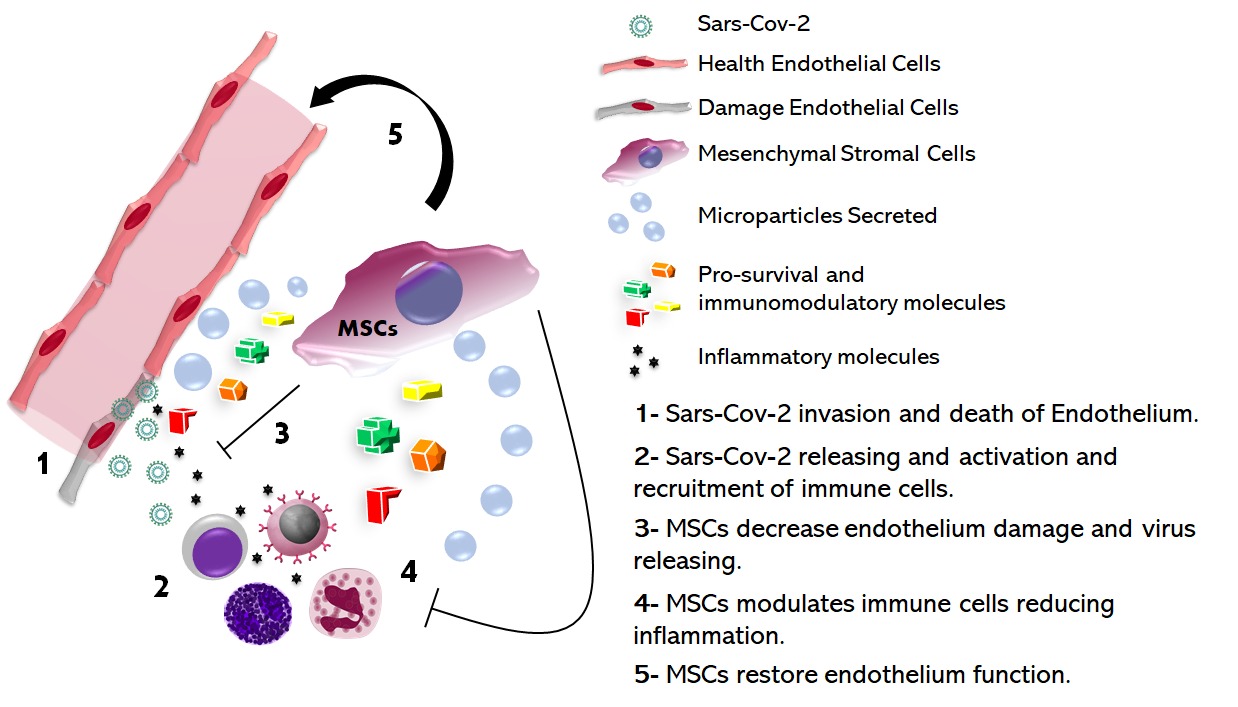
The new pandemic of SARS-CoV-2 Betacoronavirus, has spread worldwide, and infected millions of individuals causing the disease denominated of COVID-19. Further on flu symptoms, due to the high tropism of virus, has most been observed in the COVID-19 pathophysiology: acute heart failure, thromboembolism events, acute renal failure, neurological and liver damage, and multiple organ failure, with special attention to endothelial disfunction. Hence, elucidate whether virus target the endothelium is a crucial step to understand COVID-19 pathogenesis. However, the permissiveness of blood vessels during SARS-CoV-2 infection remains unclear, but regardless endothelial infection, the vascular disfunction may occurred in response to molecular inflammatory signaling triggered by immune cells that attempt to limit infection. Thus, alternative therapies using mesenchymal stromal cells (MSCs) can change this scenario and help critically ill patients. In this reflection, we attempt to discuss COVID-19 pathophysiology with impact in endothelial function and explore the applicability of MSC-based therapies as alternative treatment.
Descargas
Métricas
Citas
Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579. https://doi.org/10.1038/s41586-020-2012-7.
Tsang KW, Ho PL, Ooi GC, Yee WK, Wang T, Chan-Yeung M, et al. A Cluster of Cases of Severe Acute Respiratory Syndrome in Hong Kong. New England Journal of Medicine 2003;348. https://doi.org/10.1056/NEJMoa030666.
Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt GM, et al. A Major Outbreak of Severe Acute Respiratory Syndrome in Hong Kong. New England Journal of Medicine 2003;348. https://doi.org/10.1056/NEJMoa030685.
Cheng VCC, Lau SKP, Woo PCY, Yuen KY. Severe Acute Respiratory Syndrome Coronavirus as an Agent of Emerging and Reemerging Infection. Clinical Microbiology Reviews 2007;20. https://doi.org/10.1128/CMR.00023-07.
World Health Organization (WHO). WHO Coronavirus (COVID-19) Dashboard. WHO Covid-19 Homepage 2021. https://covid19.who.int/.
Mao R, Qiu Y, He J-S, Tan J-Y, Li X-H, Liang J, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. The Lancet Gastroenterology & Hepatology 2020;5. https://doi.org/10.1016/S2468-1253(20)30126-6.
Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. The Lancet Haematology 2020;7. https://doi.org/10.1016/S2352-3026(20)30145-9.
Spinato G, Fabbris C, Polesel J, Cazzador D, Borsetto D, Hopkins C, et al. Alterations in Smell or Taste in Mildly Symptomatic Outpatients With SARS-CoV-2 Infection. JAMA 2020;323. https://doi.org/10.1001/jama.2020.6771.
Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Internal Medicine 2020;180. https://doi.org/10.1001/jamainternmed.2020.0994.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 2020;395. https://doi.org/10.1016/S0140-6736(20)30183-5.
Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. New England Journal of Medicine 2020;382. https://doi.org/10.1056/NEJMoa2002032.
Chen Y-T, Shao S-C, Hsu C-K, Wu I-W, Hung M-J, Chen Y-C. Incidence of acute kidney injury in COVID-19 infection: a systematic review and meta-analysis. Critical Care 2020;24. https://doi.org/10.1186/s13054-020-03009-y.
de Almeida DC, Franco M do CP, dos Santos DRP, Santos MC, Maltoni IS, Mascotte F, et al. Acute kidney injury: Incidence, risk factors, and outcomes in severe COVID-19 patients. PLOS ONE 2021;16. https://doi.org/10.1371/journal.pone.0251048.
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Respiratory Medicine 2020;8. https://doi.org/10.1016/S2213-2600(20)30079-5.
Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020;323. https://doi.org/10.1001/jama.2020.5394.
Lam TT-Y, Jia N, Zhang Y-W, Shum MH-H, Jiang J-F, Zhu H-C, et al. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature 2020;583. https://doi.org/10.1038/s41586-020-2169-0.
Glowacka I, Bertram S, Muller MA, Allen P, Soilleux E, Pfefferle S, et al. Evidence that TMPRSS2 Activates the Severe Acute Respiratory Syndrome Coronavirus Spike Protein for Membrane Fusion and Reduces Viral Control by the Humoral Immune Response. Journal of Virology 2011;85. https://doi.org/10.1128/JVI.02232-10.
Hoffmann M, Kleine-Weber H, Schroeder S, KrŸger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020;181. https://doi.org/10.1016/j.cell.2020.02.052.
Fosse JH, Haraldsen G, Falk K, Edelmann R. Endothelial Cells in Emerging Viral Infections. Frontiers in Cardiovascular Medicine 2021;8. https://doi.org/10.3389/fcvm.2021.619690.
Liu F, Han K, Blair R, Kenst K, Qin Z, Upcin B, et al. SARSCoV-2 Infects Endothelial Cells In Vivo and In Vitro. Frontiers in Cellular and Infection Microbiology 2021;11. https://doi.org/10.3389/fcimb.2021.701278.
Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin–Angiotensin–Aldosterone System Blockers and the Risk of Covid-19. New England Journal of Medicine 2020;382. https://doi.org/10.1056/NEJMoa2006923.
Fosb, l EL, Butt JH, ¯stergaard L, Andersson C, Selmer C, Kragholm K, et al. Association of Angiotensin-Converting Enzyme Inhibitor or Angiotensin Receptor Blocker Use With COVID-19 Diagnosis and Mortality. JAMA 2020;324. https://doi.org/10.1001/jama.2020.11301.
Nassiri SM, Rahbarghazi R. Interactions of Mesenchymal Stem Cells with Endothelial Cells. Stem Cells and Development 2014;23. https://doi.org/10.1089/scd.2013.0419.
Saeedi P, Halabian R, Imani Fooladi AA. A revealing review of mesenchymal stem cells therapy, clinical perspectives and modification strategies. Stem Cell Investigation 2019;6:1–8. https://doi.org/10.21037/sci.2019.08.11.
Clinical Trials.gov. Federally-funded clinical studies related to COVID-19. NIH US National Library of Medicine 2021. https://clinicaltrials.gov/.
Durand N, Mallea J, Zubair AC. Insights into the use of mesenchymal stem cells in COVID-19 mediated acute respiratory failure. Npj Regenerative Medicine 2020;5. https://doi.org/10.1038/s41536-020-00105-z
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2021 Magna Scientia UCEVA

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Magna Scientia UCEVA proporciona un acceso abierto, libre y gratuito a su contenido, basado en el principio de que ofrecer al público un acceso libre a las investigaciones, ayuda a un mayor intercambio global del conocimiento. Lo cual, implica que los usuarios pueden leer, descargar, almacenar, imprimir, buscar, indexar y realizar enlaces a los textos completos de esta revista. Se permite distribuir los diversos artículos en las versiones post-print y oficial, sin previo permiso del autor o editor, considerando que el fin de este, no implica fines comerciales, ni la generación de obras derivadas; Solo se solicita la mención de la fuente así como la autoría. El titular del copyright será el o los autores que publiquen en Magna Scientia UCEVA.
Magna Scientia UCEVA está distribuida bajo los términos de la licencia https://creativecommons.org/licenses/by-nc-nd/4.0/deed.es