The power of health economics and outcomes research (HEOR) in making decisions that matter
DOI:
https://doi.org/10.54502/msuceva.v1n1a5Palabras clave:
Burden of illness (BOI), Epidemiology, Healthcare decision-making, Health economics, Outcomes researchResumen
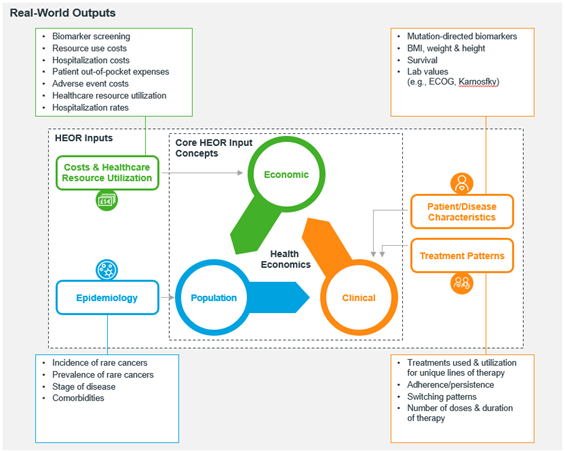
Deciding on approving and granting market access to new medical technologies such as pharmaceutical products, vaccines, or medical devices is a multifactorial research problem. Balancing out clinical performance, epidemiological implications, burden of disease, economic value, and patient preferences, among other factors, is in itself a challenging endeavor. However, this should be a mandatory requirement when making approval and market access decisions that might affect millions of people in a specific country setting. The aim of this reflection research article is twofold; first, it provides context on the important role that health economics and outcomes research (HEOR) plays in informing decision making for market access and reimbursement of new medical technologies. Second, it outlines the power of HEOR studies in guiding discussions when assessing the value of new medical technologies. Overall, this article aims at highlighting key HEOR considerations for healthcare professionals, students, and institutions interested in building analytical capabilities around this exciting and uninterruptedly growing field of knowledge.
Descargas
Métricas
Citas
Collazo Herrera M, Cárdenas Rodríguez J, González López R, Abreu RM, María Gálvez González A, Casulo JC. Temas de actualidad / Current topics La economía de la salud: ¿debe ser de interés para el campo sanitario? 1. 12. 2002. https://www.scielosp.org/pdf/rpsp/2002.v12n5/359-365/es
Santos J, Palumbo F, Molsen-David E, Willke RJ, Binder L, Drummond M, et al. ISPOR Code of Ethics 2017 (4th Edition). Value in Health 2017;20. https://doi.org/10.1016/j.jval.2017.10.018.
Hughes VS, Ferreira De Azeredo-Da-Silva AL, Hincapie AL. Health economics and outcomes research (HEOR) knowledge needs assessment for Latin America. Value in Health Regional Issues 2019;20. https://doi.org/10.1016/j.vhri.2018.10.006.
Zarate Víctor. Evaluaciones económicas en salud: Conceptos básicos y clasificación. Rev Med Chile 2010; 138:93–7. http://dx.doi.org/10.4067/S0034-98872010001000007
National Association of Pharmacy Regulatory Authorities (NAPRA). Health Canada Document: recommended guidance for community pharmacists. Ottawa, Ontario: 2019. https://napra.ca/general-practice-resources/healthcanada-document-recommended-guidance-communitypharmacists
Herrera-Restrepo O, Ruthwik Anupindi V, Wehler E, Kowal S. US healthcare databases for health economic modeling in precision medicine: An oncology-based comparative landscape assessment (CLA). Copenhagen, Denmark: 2019.
Beecroft C. Why health economics deserves a place in the medical curriculum Editorial 2016. https://doi.org/10.2217/cer-2016-0028.
International Society for Pharmaeconomics and Outcomes Research (ISPOR). The leading professional society for health economics and outcomes research (HEOR) globally. The Society’s mission is to promote HEOR excellence to improve decision making for health globally 2021. https://www.ispor.org/
Institute for Clinical and Economic Review (ICER). Fair pricing, fair access, future innovation 2021. https://icer.org/
Restrepo Zea JH, Ramírez Gómez L. Dos décadas de economía de la salud en Colombia. Cuadernos de Economía 2020;39. https://doi.org/10.15446/cuad.econ.v39n79.73067.
National Institute for Health and Care Excellence (NICE). Guidance and advice list 2021. https://www.nice.org.uk/guidance/published?type=apg,csg,cg,cov,mpg,ph,sg,sc,dg,hst,ipg,mtg,qs,ta
Alkhateeb FM, Osias K. MacKinnon III, G. Understanding Health Outcomes and Pharmacoeconomics. Burlington, MA. Jones & Bartlett Learning 2011. American Journal of Pharmaceutical Education 2012;76. https://doi.org/10.5688/ajpe76235.
World Health Organization (WHO). Burden of disease: what is it and why is it important for safer food? . 2004. https://www.who.int/foodsafety/foodborne_disease/Q%26A.pdf
World Health Organization (WHO). National burden of disease studies: a practical guide. Geneva: 2001. https://www.who.int/healthinfo/nationalburdenofdiseasemanual.pdf
University of Maryland. Steps to conducting a systematic review. University Libraries 2021. https://lib.guides.umd.edu/SR/steps
Higgins JPT T, Chandler J, Cumpston M, Li T, Page M. Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Cochrane Training 2021. https://training.cochrane.org/handbook
Berger ML, Mamdani M, Atkins D, Johnson ML. Good research practices for comparative effectiveness research: Defining, reporting and interpreting nonrandomized studies of treatment effects using secondary data sources: The ISPOR good research practices for retrospective database analysis task force report—Part I. Value in Health 2009;12. https://doi.org/10.1111/j.1524-4733.2009.00600.x.
Corey KM, Kashyap S, Lorenzi E, Lagoo-Deenadayalan SA, Heller K, Whalen K, et al. Development and validation of machine learning models to identify high-risk surgical patients using automatically curated electronic health record data (Pythia): A retrospective, single-site study. PLOS Medicine 2018;15. https://doi.org/10.1371/journal.pmed.1002701.
Berger ML, Sox H, Willke RJ, Brixner DL, Eichler H-G, Goettsch W, et al. Good practices for real-world data studies of treatment and/or comparative effectiveness: Recommendations from the joint ISPOR-ISPE Special Task Force on real-world evidence in health care decision making. Pharmacoepidemiology and Drug Safety 2017;26. https://doi.org/10.1002/pds.4297.
Schwarzer R, Rochau U, Saverno K, Jahn B, Bornschein B, Muehlberger N, et al. Systematic overview of cost–effectiveness thresholds in ten countries across four continents. Journal of Comparative Effectiveness Research 2015;4. https://doi.org/10.2217/cer.15.38.
Londoño Trujillo D, Sandoval Reyes NF, Taborda Restrepo A, Chamorro Velasquez CL, Dominguez Torres MT, Romero Ducuara SV, et al. Cost-effectiveness analysis of newborn pulse oximetry screening to detect critical congenital heart disease in Colombia. Cost Effectiveness and Resource Allocation 2019;17. https://doi.org/10.1186/s12962-019-0179-2.
Aponte-González J, Fajardo-Bernal L, Diaz J, Eslava-Schmalbach J, Gamboa O, Hay JW. Cost-Effectiveness Analysis of the Bivalent and Quadrivalent Human Papillomavirus Vaccines from a Societal Perspective in Colombia. PLoS ONE 2013;8. https://doi.org/10.1371/journal.pone.0080639.
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—Explanation and Elaboration: A Report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value in Health 2013;16. https://doi.org/10.1016/j.jval.2013.02.002.
Caro JJ, Briggs AH, Siebert U, Kuntz KM. Modeling Good Research Practices—Overview. Medical Decision Making 2012;32. https://doi.org/10.1177/0272989X12454577.
Gomez J, Moreno LE, Constenla D, Caceres D, Rodriguez E. Budget impact analysis of pneumococcal conjugate vaccines in Colombia. Expert Review of Pharmacoeconomics & Outcomes Research 2021;21. https://doi.org/10.1080/14737167.2021.1855978.
Guevara-Cuellar CA, Soto VE, Molina-Echeverry MI. Budget impact analysis of the adoption of new hypertension guidelines in Colombia. Cost Effectiveness and Resource Allocation 2018;16. https://doi.org/10.1186/s12962-018-0152-5.
Sullivan SD, Mauskopf JA, Augustovski F, Jaime Caro J, Lee KM, Minchin M, et al. Budget Impact Analysis—Principles of Good Practice: Report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value in Health 2014;17. https://doi.org/10.1016/j.jval.2013.08.2291.
Organization for Economic Cooperation and Development (OECD). The OECD and Colombia: A mutually beneficial relationship 2021. https://www.oecd.org/latin-america/countries/colombia/
International Society for Pharmaeconomics and Outcomes Research (ISPOR). Latin America Consortium. The Professional Society for Health Economics and Outcomes Research 2021. https://www.ispor.org/member-groups/global-groups/consortia/latin-america-consortium
Centers for Disease Control and Prevention (CDC). Division of Health Informatics and Surveillance 2021. https://www.cdc.gov/
Instituto Nacional de Salud (INS). Epidemiología aplicada 2021. https://www.ins.gov.co/Direcciones/Vigilancia/Paginas/Epidemiolog%C3%ADa-aplicada.aspx
Asociación Colombiana de Economía de la Salud (ACOES). Boletines ACOES. Universidad de Antioquia Facultad de Ciencias Económicas 2021. https://www.acoes.org.co/boletines
Instituto de Evaluación Tecnológica en Salud (IETS). Evaluación de efectividad y seguridad 2021. https://www.iets.org.co/Busqueda/FrmResumen.aspx?valor=Efectividad%20y%20seguridad
Universidad de Antioquia. Grupo Economía de la Salud (GES). Acerca del grupo 2021. https://www.udea.edu.co/wps/portal/udea/web/generales/interna/
Unidad Central del Valle del Cauca (UCEVA). Facultad de Ciencias de la Salud 2021. https://www.uceva.edu.co/facultad-de-ciencias-de-la-salud/
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2021 Magna Scientia UCEVA

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Magna Scientia UCEVA proporciona un acceso abierto, libre y gratuito a su contenido, basado en el principio de que ofrecer al público un acceso libre a las investigaciones, ayuda a un mayor intercambio global del conocimiento. Lo cual, implica que los usuarios pueden leer, descargar, almacenar, imprimir, buscar, indexar y realizar enlaces a los textos completos de esta revista. Se permite distribuir los diversos artículos en las versiones post-print y oficial, sin previo permiso del autor o editor, considerando que el fin de este, no implica fines comerciales, ni la generación de obras derivadas; Solo se solicita la mención de la fuente así como la autoría. El titular del copyright será el o los autores que publiquen en Magna Scientia UCEVA.
Magna Scientia UCEVA está distribuida bajo los términos de la licencia https://creativecommons.org/licenses/by-nc-nd/4.0/deed.es